Q) Sketch & describe the Reverse Osmosis system for the production of drinking water on board the ships.
Reverse Osmosis:-
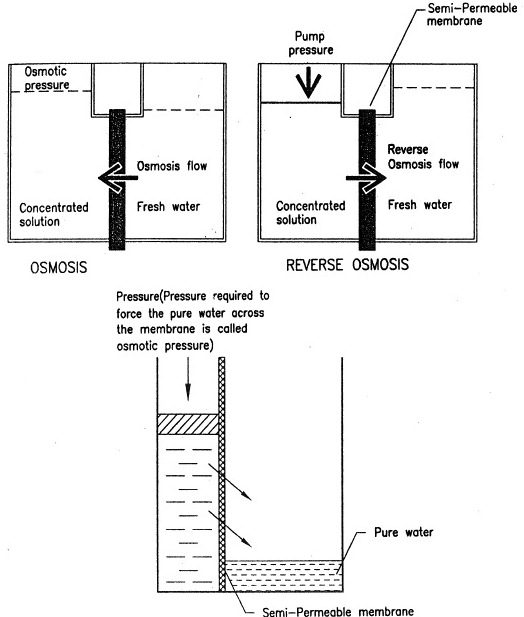
- One of the methods of producing fresh water is by using the principle of Osmosis. Osmosis term is used to describe passage of pure water from one side off a semi-permeable membrane into a salt or other solution on the other, with the result the salt solution is diluted but the water remains pure.
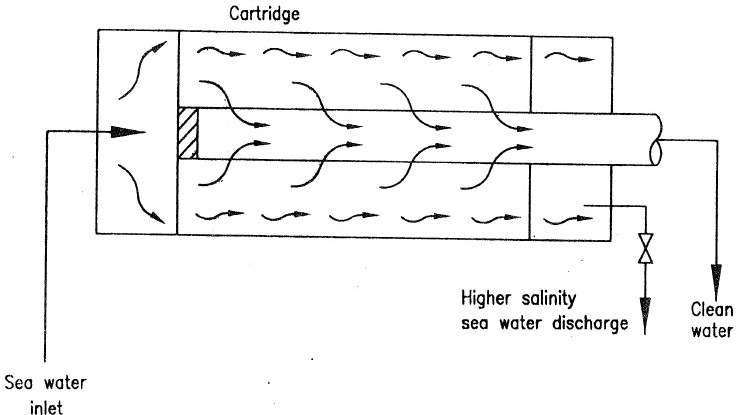
- Reverse Osmosis is a water filtration process, which makes use of semi-membrane materials. Salt water on one side of the membrane is pressurized by a pump and forced against the material. Pure water passes through but not thee salts see figure 1.
- Pressure required to force the pure water through, is called osmotic pressure & requirement of osmotic pressure is higher if the salinity of salt water is higher. For production of large amounts of pure water, the membrane area must be large and it must be tough enough to withstand the pump pressure.
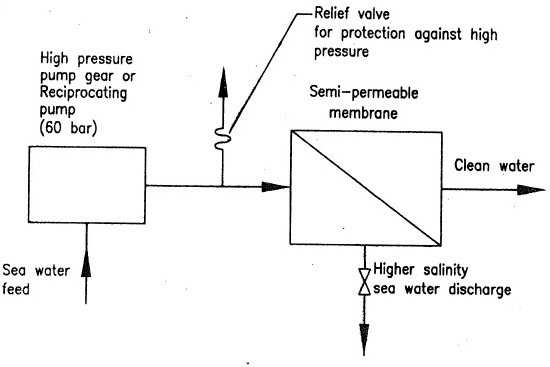
- Also, as continuous supply of sea water is used as feed, the salinity of feed is steady, than the osmotic pressure required to force fresh water through is also steady, and steady pressure is taken from a continuously running pump, see figure 2. Semi permeable membrane may be provided by a bunch off cartridge as shown in figure 3.
Q) State – Pre-treatment used with RO equipment.
Ans:- Due to the low temperature of operation in low pressure evaporators, the fresh water produced by such a FWG may be harmful to drink as it may contain bacteria. It may not even contain the minerals that are needed. Therefore, it needs to be treated, in order for it to be potable. The treatment of fresh water can be done using the following methods:
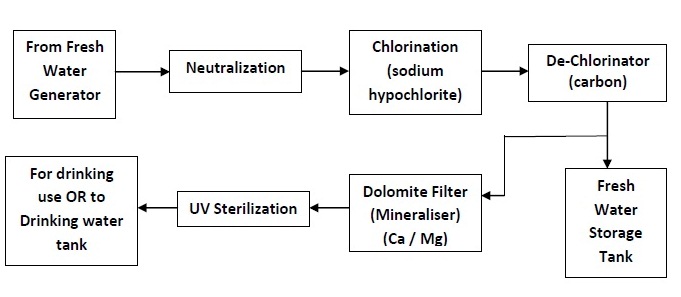
- Chlorine Sterilization: The sterilization by chlorine is recommended and it may be done by an excess dose of chlorine provides by sodium hypochlorite in a liquid form or by adding calcium chloride granules. The chlorine content may be upto 0.2 ppm, for it to be effective. The water is then de-chlorinated in a bed of activated carbon to remove the excess chlorine. Any colour, taste and odour, which were present in the water, will also be removed by the carbon.
- Silver Ion Sterilization: In this method, silver ions are injected into the distillate, by means of a silver anode. This method of sterilization is effective since silver is toxic to the bacteria present in the water. Unlike chlorine, the silver ions do not evaporate. The amount of silver ion released into the water is controlled by the current and the silver ion content may be upto 0.08 ppm, for it to be effective.
- Ultraviolet Light Sterilization: A temporary but immediately effective sterilization is by means of UV light. Chlorine and silver ion methods although long lasting, change the taste of the water and require efficient carbon filtration to remove the odour. UV light on the other hand does not cause any physical or chemical change in the water. This method uses UV lamps to produce short wave radiations that destroy the bacteria, viruses and other organisms in the water. This method is usually used on the discharge side of the water storage tank so that the water is sterilized immediately before use.
- Sterilization by Ozone: Use of ozone for sterilization is very effective. It is an effective oxidant. However, the equipment is costly and has high running cost.
An example of treatment of fresh water produced by the FWG is shown in the flowchart below. The system may be different on different ships. The end result should however be that the produced water made available for drinking is slightly alkaline, sterilized, clear and good in taste.
Q) State – The Post-treatment necessary.
- The Permeate (product water) from the above process may contain small quantities of dissolved salts and is suitable as drinking water if the salt content is below 250 ppm, well within the WHO limit.
- However, if the water is to be used for boiler feed, it has to be completely free from salts and the product water may require further treatment to get rid of small quantities of salts by Ion Exchange Treatment.